Our research
Overview: Organelle biogenesis and quality control
Our lab’s long-term research interest is to understand molecular mechanisms by which eukaryotic intracellular organelles, such as endoplasmic reticulum and mitochondria, are generated and their functions are maintained. Currently, our research focuses on (1) transport of newly synthesized polypeptides across the membranes of organelles, (2) quality control pathways that recognize dysfunctional proteins and organelles, and (3) transport proteins that are important in maintaining organelles’ functions, and (4) involvements of these factors and processes in human diseases. To address these issues, we use a combination of biochemistry, biophysics, and structural biology.
Protein translocation across the endoplasmic reticulum membrane
Endoplasmic reticulum (ER) is one of the most abundant organelles in eukaryotic cells and the major site of the protein biosynthesis. In most organisms, about 30% proteins are either membrane proteins or soluble proteins that are transported via the secretory pathway (such as extracellular proteins, and proteins in the lumens of ER, Golgi, and lysosomes). These proteins must be first transported across or integrated into the ER membrane before targeted to other subcellular locations via vesicular trafficking. This process, so-called protein translocation, is mediated by the universally conserved Sec61 protein-conducting channel, also known as the translocon. Prokaryotes also use the homologous complex SecY channel to secrete soluble proteins across or integrate membrane proteins into the plasma membrane.
The Sec61/SecY complexes have been extensively studied in the past, but several key mechanistic aspects remain incompletely understood. One such area is post-translational protein translocation. While a majority of proteins are translocated across the ER membrane co-translationally by Sec61 docking with a ribosome-nascent-chain complex, many proteins are targeted to Sec61 post-translationally. This is either because these polypeptides are too short to be targeted co-translationally by the signal recognition particle (SRP) or because their N-terminal targeting signals (signal sequences) cannot be efficiently recognized by the SRP. Post-translational translocation requires the two additional membrane proteins Sec62 and Sec63, which are stably associated with the Sec61 channel, forming a machinery called the Sec complex. Although Sec62 and Sec63 have been known as essential factors for post-translational translocation for 30 years, their functions had been elusive.
Recently, our lab has determined high-resolution structures of the Sec complex from two fungal species Saccharomyces cerevisiae and Thermomyces lanusinosus using cryo-electron microscopy (cryo-EM) (Itskanov and Park, Science, 2019; Itskanov et al., Nat Struct Mol Biol, 2021). Our first Sec complex structure in the 2019 paper revealed the architecture of the Sec complex and proposed that Sec63 activates the Sec61 channel to facilitate engagement with a post-translational substrate polypeptide. Our 2021 paper further polished this model for the mechanism of the Sec complex. The Sec61 channel has two gating mechanisms: a vertical pore for translocation of soluble peptides and a lateral gate for membrane insertion of signal sequences (or transmembrane helices). Both gates are closed in the resting state but were expected to open only during protein translocation. We found that surprisingly, in the Sec complex, both gates are opened by Sec63 and Sec62 even in the absence of a polypeptide substrate. Sec63 opens the lateral gate, and Sec62 further opens the vertical pore. Our mutagenesis studies suggest that this pre-opening of the Sec61 channel is critical for translocation of post-translational substrates because otherwise the channel cannot efficiently engage with these substrates. Furthermore, the data suggests that Sec62 would also be important in preventing the channel from being clogged by lipids infiltrating through the open lateral gate.

Mitochondrial protein import
The mitochondrion is the cell’s powerhouse that generates adenosine triphosphate (ATP) through oxidative phosphorylation. The organelle also performs other various vital functions, such as cell signaling, biosynthesis of heme and Fe-S clusters, and apoptosis. These functions are carried out through the coordinated activities of ~1,000 to 1,500 proteins in mitochondria. Although mitochondria have their own genome, the vast majority (99%) of mitochondrial proteins are encoded by nuclear DNA and imported into the organelle mainly in a post-translational manner. Mitochondrial protein import is thus one of the most important steps in mitochondrial biogenesis.
The mitochondrion is a double-membrane-bound organelle, which contains four distinct sub-organellar spaces: the outer membrane (OM), the inner membrane (IM), the intermembrane space (IMS), and the matrix. This complex organellar structure as well as the structural and topological diversity of proteins that are targeted to mitochondria necessitates elaborate membrane protein machineries to enable protein import processes. Among those, the best-known import machineries are the TOM complex in the OM and the TIM23 complex in the IM. About 90% mitochondrial proteins are first transported across the OM through the TOM complex. The TIM23 complex mediates further translocation of matrix proteins across the IM and integration of most IM membrane proteins into the IM. These processes pose fascinating biological questions: how are pores for protein translocation formed, what drives protein translocation across the membranes, and how do the translocation machineries interact with precursor proteins to dictate targeting to specific compartments in mitochondria? These questions remain poorly understood, hampered by scarcity of structural information on the import machineries.
Our group has recently elucidated high-resolution cryo-EM structures of the TOM complex from S. cerevisiae (Tucker and Park, Nat. Struct. Mol Biol., 2019). These structures revealed the organization of five different TOM subunits and the architecture of the protein translocation pore, which are expected to be conserved in all species including humans. Our structural and mutagenesis analyses provided a mechanistic model for how the TOM complex might recognize and engage with substrate polypeptides, particularly those with an N-terminal mitochondrial-targeting signal (presequence). Our current model is that an electrostatic interaction between the negatively charged pore and positively charged presequences provides an important driving force for initial substrate engagement. Our cryo-EM structures also suggest that dimeric TOM complexes may form higher-order oligomeric structures, the functions of which remain to be elucidated.

Endoplasmic reticulum protein quality control
Identities and functionalities of organelles depend on correct localization of proteins. The endoplasmic reticulum (ER) and mitochondrial membranes are the primary destinations for newly-synthesized membrane proteins with hydrophobic transmembrane segments. But what determines the destination of these membrane proteins is poorly understood. Why some membrane proteins end up in mitochondria, whereas other membrane proteins end up in the ER? The answer to this question has been elusive because the targeting signals are the transmembrane segments themselves, which all share the same biophysical property (i.e., hydrophobicity) regardless of subcellular locations.
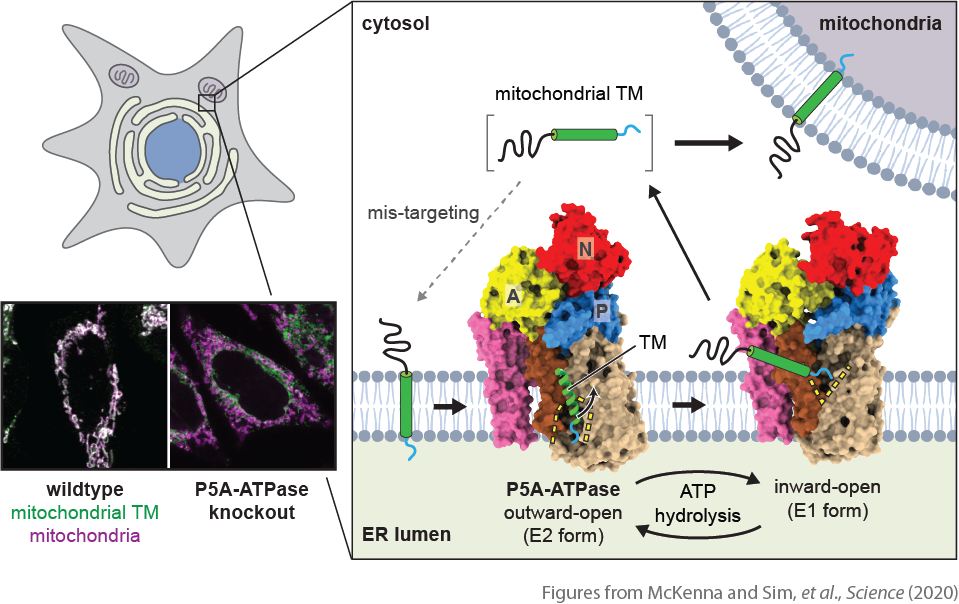
One example is targeting of tail-anchored (TA) membrane proteins, which contain a single transmembrane helix near the very C-terminus of the polypeptide. Both the ER and mitochondrial outer membranes contains distinct sets of TA proteins, which are targeted into the membranes by a post-translational mechanism. At the ER, the insertion is mediated by the GET or EMC complex. The mechanism of TA protein insertion into the mitochondrial OM is currently unknown. Nevertheless, whether it is ER-targeted or mitochondrial-targeted, their C-terminal transmembrane helices are not significantly distinctive from each other. In fact, several papers have documented that in certain genetic backgrounds, TA proteins are often mistargeted to wrong organelles.
In collaboration with Sichen Shao’s lab at Harvard Medical School, we have recently identified a novel quality control mechanism that is crucial for high-fidelity protein localization at the ER and mitochondrial outer membranes (McKenna, Sim et al., Science, 2020). We discovered that an orphan P-type ATPase transporter at the ER, known as P5A-ATPase, plays a critical role in correcting localization of TA proteins. In the absence of the P5A-ATPase, many mitochondrial TA proteins abnormally accumulate at the ER. Our structural analysis of the yeast P5A-ATPase (Spf1) and biochemical studies of the mammalian P5A-ATPase (ATP13A1) revealed that the P5A-ATPase dislocates mis-localized mitochondrial TA proteins from the ER membrane. With high-resolution cryo-EM structures, we showed that the P5A-ATPase contains a large pocket that can accommodate a short hydrophilic segment after the transmembrane helix of substate TA proteins. By alternating the opening of this pocket, the P5A-ATPase extracts mistargeted TA proteins from the membrane. The pocket is negatively charged, suggesting that it recognizes mitochondrial TA proteins through interactions with positive charges in the hydrophilic segment—the main characteristic of mitochondrial TA proteins the importance of which was previously enigmatic. Proteomic analysis also indicates that the P5A-ATPase also extracts mis-inserted signal sequences of ER-targeted proteins from the membrane. These findings explain pleiotropic ER dysfunction phenotypes caused by the P5A-ATPase disruption, which have been mysterious for years.